
 04/22/2020
04/22/2020Support our Covid-19 work
Support Oxford’s important work in coronavirus research by making a donation. Academics from around the University are working on a...Read postRead post
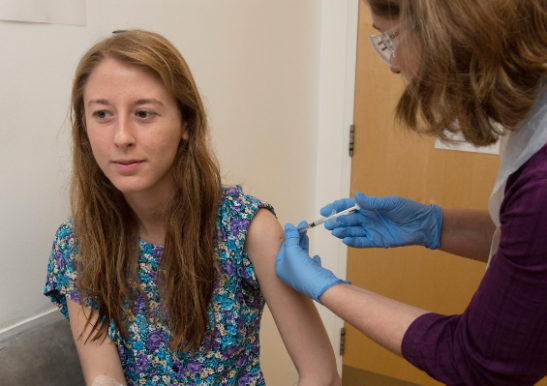
The Phase III interim results of the Oxford and AstraZeneca vaccine trials have just been published. The results show that the vaccine is effective at preventing Covid-19, with overall 70.4% efficacy. Excitingly in one dosing regimen, when people received a half dose first followed up with a second full dose, the vaccine demonstrated 90% efficacy. If this dosing regimen is used it means that more people can be vaccinated with the planned vaccine supply.
There were no hospitalised or severe cases in anyone who received the vaccine.
The Oxford Vaccine is made from a weakened version of a common cold virus (adenovirus), that has been genetically changed so that it is impossible for it to grow in humans. Adenovirus vaccines have been researched and used extensively, and their safety tracked for decades. They have the significant benefit of being easily manufactured, transported and stored at domestic fridge temperature allowing for rapid and lower cost deployment around the world, once approved.
A key element of Oxford’s partnership with AstraZeneca is the joint commitment to provide the vaccine on a not-for-profit basis for the duration of the pandemic across the world, and in perpetuity to low- and middle- income countries.
Professor Louise Richardson, Vice-Chancellor of the University of Oxford said, “This is a great day for the University of Oxford and for universities everywhere. Pushing at the frontiers of knowledge with partners across the globe and putting our extraordinary brainpower in service to society, is what we do best.”
You can find the official announcement on this news on our website here and there is a short video about the vaccine in this link.